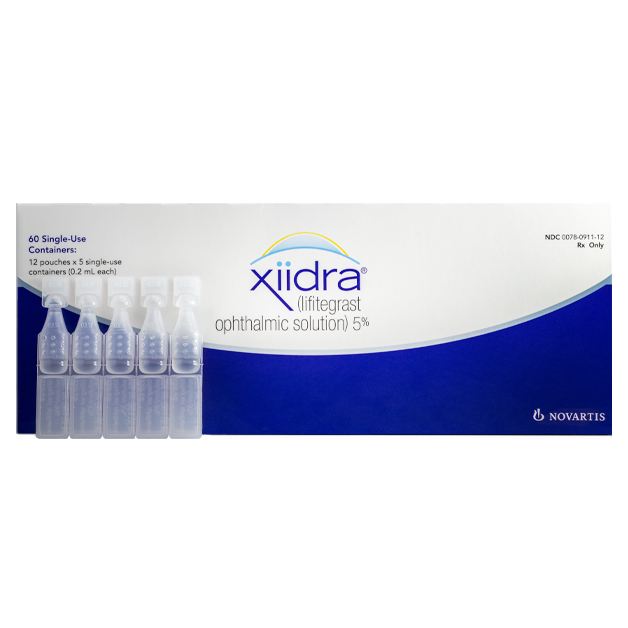
Contact Us
Patient Resources
Eye Care Professionals
Search
 United States
United States
 United States
United States
Countries/Regions/Markets
- Argentina
- Australia
- Austria / Österreich
- Belgium / België / Belgique / Belgien
- Brazil / Brasil
- Canada / English
- Canada / Français
- China / 中国
- Croatia / Hrvatska
- Czech Republic / Česká republika
- Denmark / Danmark
- Finland / Suomen tasavalta
- France / République française
- Germany / Deutschland
- Greece / Ελλάδα
- India / Bhārat
- Indonesia
- Ireland / Éire
- Italy / Italia
- Japan / 日本
- Korea / 한국 / 대한민국
- Latvia / Latvija
- Lithuania / Lietuva
- Malaysia
- Netherlands / Nederland
- New Zealand
- Norway / Norge / Noreg
- Pakistan / پاکستان
- Philippines / Pilipinas
- Poland / Polska
- Portugal
- România
- Russia / Росси́я
- Singapore
- Slovakia / Slovenská
- Slovenia / Slovenija
- South Africa
- Spain / España
- Sweden / Sverige
- Switzerland / Schweiz / Svizzera
- Taiwan / 台湾
- Thailand / ราชอาณาจักรไทย
- Türkiye
- Ukraine / Україна
- United Kingdom (UK)
- United States of America (USA)
Countries/Regions/Markets:
- Argentina
- Australia
- Austria / Österreich
- Belgium / België / Belgique / Belgien
- Brazil / Brasil
- Canada / English
- Canada / Français
- China / 中国
- Croatia / Hrvatska
- Czech Republic / Česká republika
- Denmark / Danmark
- Finland / Suomen tasavalta
- France / République française
- Germany / Deutschland
- Greece / Ελλάδα
- India / Bhārat
- Indonesia
- Ireland / Éire
- Italy / Italia
- Japan / 日本
- Korea / 한국 / 대한민국
- Latvia / Latvija
- Lithuania / Lietuva
- Malaysia
- Netherlands / Nederland
- New Zealand
- Norway / Norge / Noreg
- Pakistan / پاکستان
- Philippines / Pilipinas
- Poland / Polska
- Portugal
- România
- Russia / Росси́я
- Singapore
- Slovakia / Slovenská
- Slovenia / Slovenija
- South Africa
- Spain / España
- Sweden / Sverige
- Switzerland / Schweiz / Svizzera
- Taiwan / 台湾
- Thailand / ราชอาณาจักรไทย
- Türkiye
- Ukraine / Україна
- United Kingdom (UK)
- United States of America (USA)